Structure and Properties - Flake Graphite
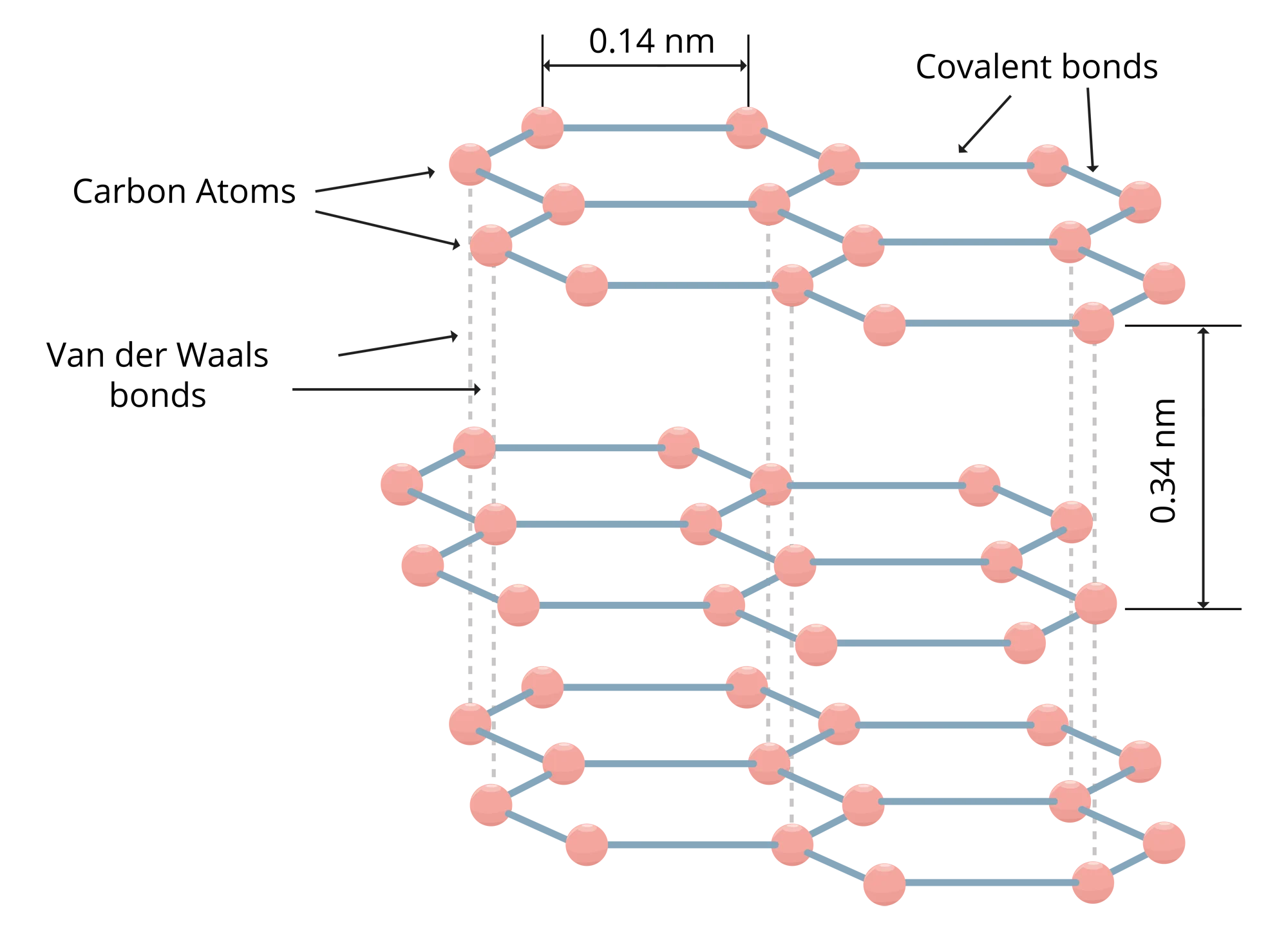
Flake graphite has a layered structure constituting carbon atoms in a hexagonal lattice. Each planer layer constitutes carbon atoms with sp2 configuration, each carbon atom is bonded to three other carbon atoms via covalent bonds, leaving one free electron each. These planer sheets, one sheet is called graphene, are weakly bonded via Van der Waals force giving the flaky nature to the material. Given these molecular characteristics, flake graphite is a material with a unique set of properties, making it the preferred and mostly non-replaceable choice in various industries, products and applications. Though it is a non-metal, it has unique properties of both metals and non-metals.
As a Conductor
- Being a pure form of carbon, it is the only non-metallic high-performance conductor of electricity
- It is also the only non-metallic high-performance conductor of heat
Given the above two properties, flake graphite is the only known material occurring in a free-flowing powder which is also a good conductor, making it the material of choice in various applications, including energy storage.
Structural Properties
- The mirror finish flakes of graphite align on any surface providing excellent solid state lubrication, that can sustain higher temperature as compared to petroleum based lubricants
- It is a reflector of light, giving it a shining lustre
- Infusions of certain chemicals within the layers of flake graphite, a process referred to as intercalation, weaken the vertical Van der Waals bonds in crystals. This instils a property of expansion on heating, which can exceed 300 times in volume. Commonly, this is known as Expandable Graphite, a key material in flame retardants.
Chemical & Other Properties
- With melting temperature exceeding 3000° Centigrade, flake graphite is an excellent refractory material in reducing atmosphere
- Negligible coefficient of thermal expansion, an added advantage to its refractory properties, a property exhibited by very few known materials
- Chemically inert and non-hazardous
- Low adsorption of X-rays and neurons